Полониум
84
Po
Група
16
Периода
6
блок
p
Протони
Електрони
Неутрони
84
84
126
Општи Својства
Атомски број
84
Атомска тежина
[210]
Масен број
210
Категорија
Металоид
Боја
Сребро
Радиоактивно
Да
Named after Poland, native country of Madam Curie
Кристална структура
Обично кубно
Историја
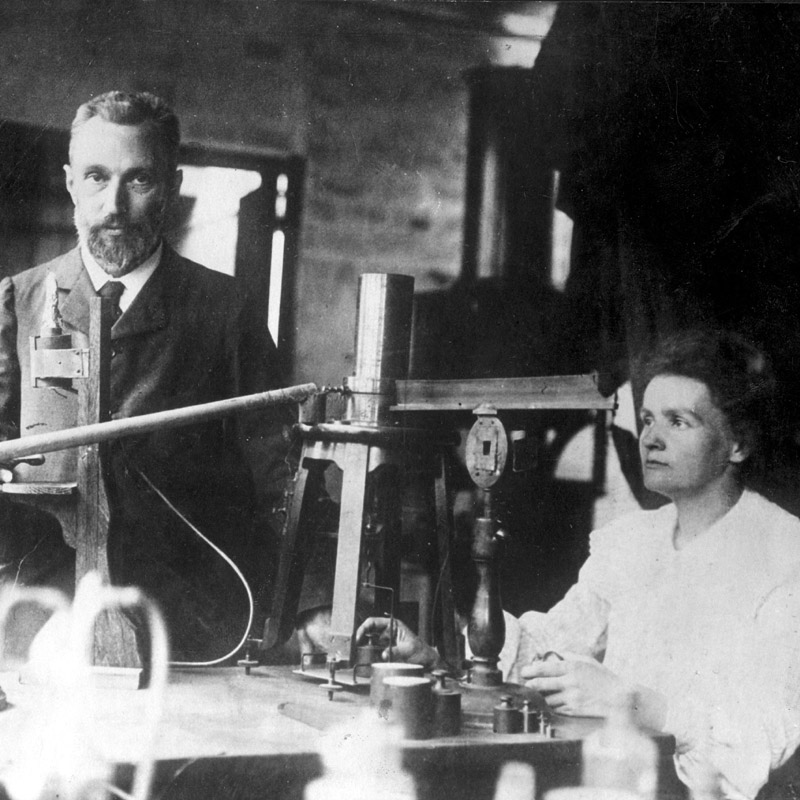
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Електрони на орбитала
2, 8, 18, 32, 18, 6
Електронска конфигурација
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Физички Својства
Фаза
Цврста состојба
Густина
9,196 g/cm3
Точка на топење
527,15 K | 254 °C | 489,2 °F
Точка на вриење
1235,15 K | 962 °C | 1763,6 °F
Топлина на топење
13 kJ/mol
Топлина на испарување
100 kJ/mol
Специфичен топлински капацитет
- J/g·K
Обилност во Земјината кора
Нема одговор
Обилност во Универзумот
Нема одговор
CAS број
7440-08-6
PubChem CID број
Нема одговор
Атомски Својства
Атомски полупречник
168 pm
Ковалентен полупречник
140 pm
Електронегативност
2,00 (Полингова скала)
Јонизациски потенцијал
8,417 eV
Атомски волумен
22,23 cm3/mol
Топлинска спроводливост
0,2 W/cm·K
Оксидациски состојби
-2, 2, 4, 6
Примени
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Изотопи
Стабилни изотопи
-Нестабилни изотопи
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po