Радиум
88
Ra
Група
2
Периода
7
блок
s
Протони
Електрони
Неутрони
88
88
138
Општи Својства
Атомски број
88
Атомска тежина
[226]
Масен број
226
Категорија
Земноалкален метал
Боја
Сребро
Радиоактивно
Да
From the Latin word radius meaning ray
Кристална структура
Телоцентрирана кубна
Историја
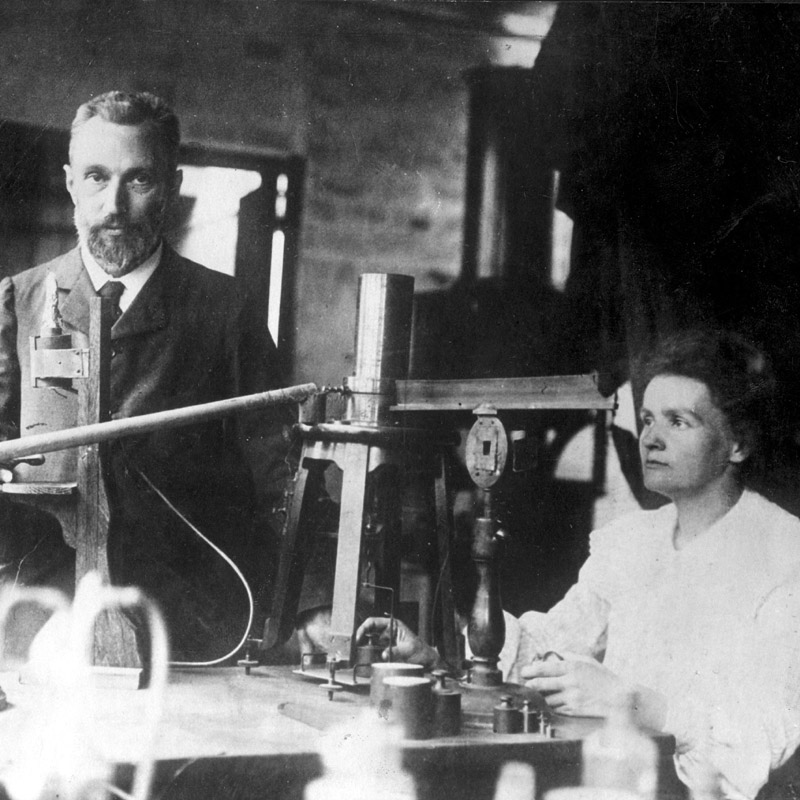
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Електрони на орбитала
2, 8, 18, 32, 18, 8, 2
Електронска конфигурација
[Rn] 7s2
Radium imparts a carmine red color to a flame
Физички Својства
Фаза
Цврста состојба
Густина
5,5 g/cm3
Точка на топење
973,15 K | 700 °C | 1292 °F
Точка на вриење
2010,15 K | 1737 °C | 3158,6 °F
Топлина на топење
8 kJ/mol
Топлина на испарување
125 kJ/mol
Специфичен топлински капацитет
- J/g·K
Обилност во Земјината кора
9,9×10-12%
Обилност во Универзумот
Нема одговор
CAS број
7440-14-4
PubChem CID број
6328144
Атомски Својства
Атомски полупречник
-
Ковалентен полупречник
221 pm
Електронегативност
0,9 (Полингова скала)
Јонизациски потенцијал
5,2784 eV
Атомски волумен
45,20 cm3/mol
Топлинска спроводливост
0,186 W/cm·K
Оксидациски состојби
2
Примени
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Изотопи
Стабилни изотопи
-Нестабилни изотопи
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra